Case Publication Report: Malignant Ascites and Pleural Effusion in Stage 4 Ovarian Carcinoma – An Elaborated Case
Thanu P S1, Gowdham P2
Department of Nursing, Kauvery Hospital, Marathahalli
Abstract
This case report presents a detailed account of a 65-year-old female diagnosed with Stage 4 ovarian carcinoma, metastatic peritoneal carcinomatosis, and left lower extremity deep vein thrombosis, who experienced acute exacerbation of breathlessness. Her presentation was primarily driven by massive malignant ascites and bilateral pleural effusions. Despite initial symptomatic interventions including therapeutic ascitic tapping and pigtail catheter drainage, her complex medical condition necessitated a multi-faceted approach to medical management and palliative care. This report aims to highlight the intricate interplay of disease progression, symptomatic management, and the crucial role of nursing care in advanced oncological settings.
Case Presentation
A 65-year-old female, presented to the emergency room with the chief complaint of progressive breathlessness for five days, significantly aggravated in the supine position over the preceding two days. She reported no associated chest pain, fever, vomiting, or loose stools. Her significant past medical history includes Stage 4 ovarian carcinoma, diagnosed with metastatic peritoneal carcinomatosis, and a history of left lower extremity deep vein thrombosis (DVT). The patient’s family history indicated no known genetic predisposition to cancer.
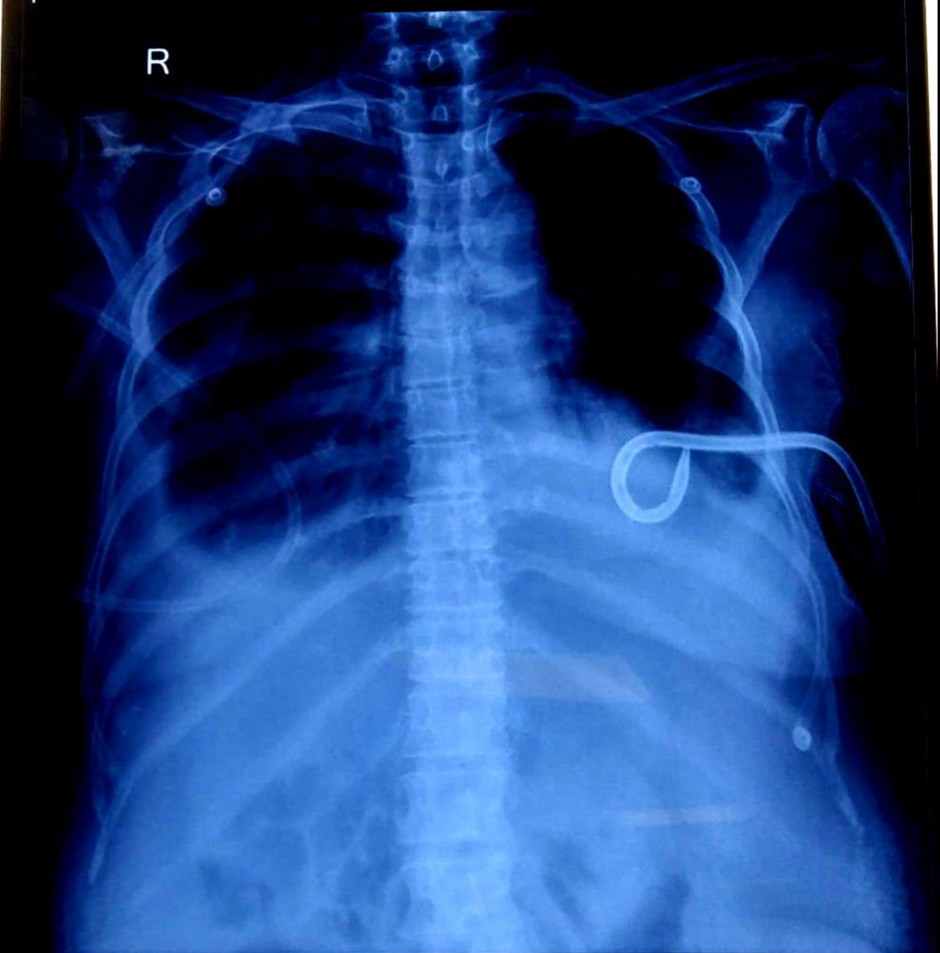
Prior to the current admission, she had undergone a pigtail catheter insertion on June 9th, 2025, at 7:00 PM, for the management of refractory ascites. Drain outputs after this procedure were substantial: 1200 ml on June 10th, 2025, and 465 ml on June 11th, 2025. A unit of Packed Red Blood Cells (PRBC) was transfused on June 10th, 2025, to address anaemia. She had a long-standing history of abdominal distension, with a recorded maximum girth of 92 cm, for which recurrent ascitic tapping had been performed. Despite recommendations for systemic chemotherapy, the patient had previously opted to decline active oncological treatment, indicating a preference for palliative and symptomatic management.
Investigations
Imaging Studies:
- Echocardiography (March 3, 2025, and June 9, 2025): The initial echocardiogram demonstrated concentric left ventricular hypertrophy (LVH), mild tricuspid regurgitation (TR), absence of regional wall motion abnormalities (RWMA), and preserved left ventricular systolic function. Left-sided pleural effusion was also noted. A subsequent transthoracic echocardiography on June 9, 2025, confirmed persistent tachycardia (HR: 103 bpm), adequate LV systolic function (ejection fraction 55%), Grade I LV diastolic dysfunction, trivial mitral regurgitation (MR), mild TR, no significant pulmonary arterial hypertension (PAH) at rest, and importantly, the continued presence of pleural effusion. Cardiac chambers and septae were structurally normal.
- CT scan of Abdomen & Pelvis (March 2, 2025): Revealed moderate ascites, interbowel fluid, clumped bowel loops, extensive omental caking (omental thickness 42mm), and diffuse peritoneal thickening, consistent with peritoneal carcinomatosis. Mild bilateral pleural effusion (right > left) was additionally identified. The report suggested ruling out abdominal tuberculosis (Koch’s) and recommended ascitic fluid analysis and Adenosine Deaminase (ADA) testing.
- Ultrasound of Abdomen & Pelvis (February 28, 2025): Confirmed gross ascites, bilateral pleural effusion (right mild, left minimal), and a thickened/edematous gallbladder wall.
- F18 FDG PET CT Scan (March 10, 2025): This scan was performed considering her ascites and a positive pleural fluid cytology for adenocarcinoma. It showed moderate to large ascites, significant omental thickening/nodularity with low-grade heterogeneous FDG uptake (maximum SUV 6.4, thickness up to 2.5 cm), and mild peritoneal surface thickening, indicative of active malignant disease. A small right pleural effusion with low-grade FDG uptake (SUV 4-5) and trace left pleural effusion were observed, without definitive enhancing or FDG avid pleural nodularity. Passive subsegmental collapse of the right lower lobe and streaky fibrotic changes were also noted. There was no definite enhancing or FDG avid mass lesion identified in the ovaries, which appeared normal in size.
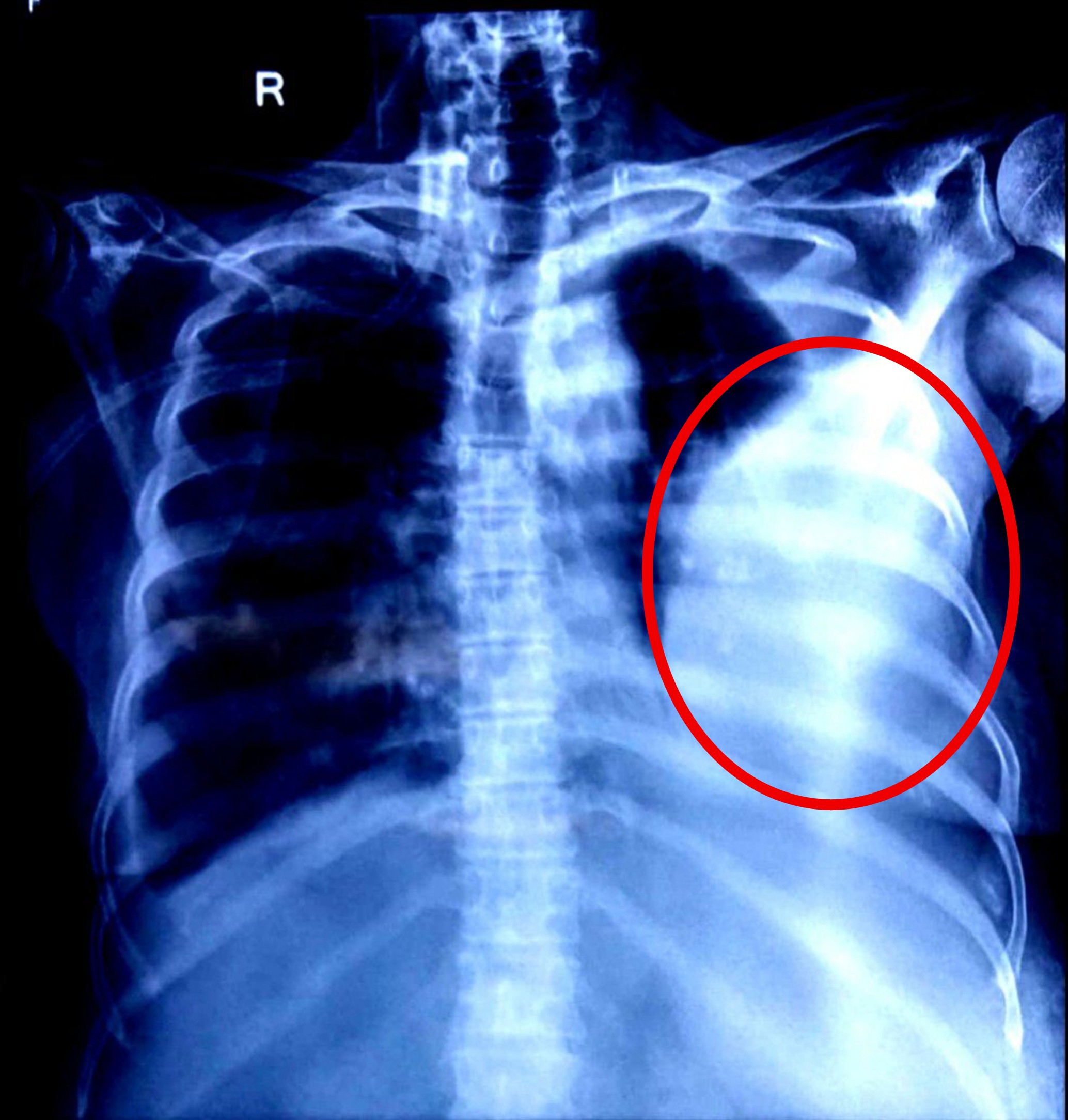
- Chest X-ray AP (June 9, 2025, and June 11, 2025): Both radiographs consistently demonstrated the presence of significant pleural effusion, correlating with her respiratory symptoms.
Laboratory Investigations:
- Complete Blood Count (June 9, 2025): Notable findings included mild anemia (Hemoglobin: 9.35 g/dl, Hematocrit: 27.3%, MCV: 68.6 fl, MCH: 23.5 pg, RDW-CV: 21.6), and elevated platelet count (466.0 x10^3/uL), possibly reactive. Total WBC count was within the normal range.
- Liver Function Test (June 9, 2025): All bilirubin fractions and liver enzymes (SGOT, SGPT, Alkaline Phosphatase, GGT) were within normal limits. However, total protein (5.7 g/dl) and albumin (2.0 g/dl) were low, with a consequently inverted A/G ratio (0.540), indicative of malnutrition and chronic disease.
- Pleural Fluid Analysis (June 9, 2025): The aspirated 45 ml of reddish, turbid fluid showed 950 WBCs and 6000 RBCs. Cytological analysis indicated features “suspicious for malignant cells,” further supporting the metastatic nature of her effusions.
- Renal Function Test (June 11, 2025): Urea and creatinine levels were within normal limits, suggesting preserved renal function. Electrolytes showed mild hyponatremia (Sodium: 132 mmol/L), while potassium and chloride were within range.
- Repeat CBC (June 11, 2025): Hemoglobin remained low at 9.90 g/dl, with persistent microcytic and anisocytic features (MCV: 74.3 fl, RDW-CV: 24.3). Platelet count remained elevated (3730 x10^3/uL), and a mild leukopenia (3.77 x10^3/uL) was noted.
Medical Management
Given the patient’s refusal of chemotherapy and her advanced disease stage, medical management focused primarily on symptomatic control and supportive care. Her treatment regimen included:
- Intravenous (IV) Antibiotics:
- Inj. Ceftriaxone 1 gm IV BID: A broad-spectrum cephalosporin, likely administered to prevent or treat potential infections, especially given the invasive procedures (pigtail insertion, ascitic tapping) and immunocompromised state due to advanced malignancy and malnutrition.
- Gastrointestinal Protection:
- Inj. Pantoprazole 40 mg IV OD: A proton pump inhibitor used to reduce gastric acid secretion, protecting against stress ulcers or reflux, which can be common in critically ill or oncological patients.
- Pain and Fever Management:
- Inj. Paracetamol 1 gm IV QID: Administered intravenously for pain and fever control, offering systemic and symptomatic relief.
- Inj. Tramadol 50 mg in 100 ml NS (normal saline) started: An opioid analgesic for moderate to severe pain relief, often used in cancer pain management. The infusion method provides sustained pain control.
- Anti-emetic:
- Inj. Emeset 4 mg IV SOS (as needed): An antiemetic used to manage nausea and vomiting, which can arise from the malignancy itself, concurrent medications, or physiological distress.
- Anticoagulation:
- Inj. Heparin 5000 IU Subcutaneous (SC): Administered for thromboprophylaxis or treatment of her pre-existing left lower extremity DVT. Patients with advanced malignancies are at significantly increased risk of venous thromboembolism due to hypercoagulability.
- Nutritional and Volume Support:
- Inj. Albumin 20% 100 ml over 5 hours, started: Administered to address her hypoalbuminemia. Albumin infusions can help in maintaining oncotic pressure, potentially reducing fluid extravasation into the third spaces (ascites, pleural effusion), and providing nutritional support.
- Pulmonary Support:
- Chest Physiotherapy and Spirometry: Initiated to improve lung function, facilitate secretion clearance, and enhance respiratory muscle strength, crucial for managing breathlessness caused by effusions.
Discussion
Mrs. XXXX’s presentation vividly illustrates the complex pathophysiology of advanced ovarian carcinoma with metastatic spread. Her breathlessness, the cardinal symptom, can be attributed to the combined effects of gross malignant ascites and bilateral malignant pleural effusions.
Anatomy and Physiology of Malignant Effusions: The peritoneum, a serous membrane, normally maintains a delicate balance of fluid production and reabsorption. In peritoneal carcinomatosis, malignant cells infiltrate the peritoneum, causing increased vascular permeability, direct fluid secretion by tumor cells, and crucially, obstruction of the lymphatic drainage pathways (e.g., stomata in the diaphragm and peritoneal lymphatics). This disrupts the homeostatic mechanism, leading to the rapid and excessive accumulation of malignant ascites. The significant volume of fluid, as evidenced by the 92 cm abdominal girth and large drain output, elevates intra-abdominal pressure. This pressure mechanically impedes the downward excursion of the diaphragm, directly compromising lung expansion and leading to restrictive ventilatory defects, manifested as breathlessness, particularly orthopnea.
Similarly, the pleura, comprised of visceral and parietal layers, encloses the lungs. Malignant pleural effusions result from the dissemination of cancer cells to the pleural surfaces, leading to increased fluid transudation, impaired lymphatic clearance through blocked stomata, and sometimes direct tumor growth on the pleura. The presence of pleural fluid compresses the underlying lung parenchyma, reducing vital capacity and exacerbating dyspnea. The PET CT findings with FDG uptake in the pleural effusion underscore the metabolic activity of the metastatic cells contributing to the effusion.
The patient’s persistent hypoalbuminemia is a critical factor. Albumin plays a vital role in maintaining plasma oncotic pressure. Low albumin levels reduce this pressure, leading to fluid shifts from the intravascular space into interstitial and third spaces, thereby contributing to the formation and persistence of both ascites and pleural effusion. The albumin infusion aims to counteract this physiological imbalance.
The patient’s refusal of active chemotherapy, within her autonomous rights, underscores the shift towards palliative care. The medical management regimen reflects this approach, focusing on symptomatic relief (dyspnea, pain, nausea) and addresses immediate complications (anemia, DVT risk, electrolyte imbalance). The use of antibiotics suggests a proactive approach to potential infection risk associated with advanced cancer and invasive procedures. The addition of chest physiotherapy and spirometry demonstrates a comprehensive approach to respiratory support, aiming to optimize lung function and alleviate her breathlessness.
Nursing Diagnoses
Based on the comprehensive assessment, the following nursing diagnoses are pertinent to Mrs. XXXX’s care:
1. Impaired gas exchange related to altered oxygen supply (pleural effusion, ascites compressing diaphragm) and hypoventilation as evidenced by breathlessness, orthopnea, and abnormal chest X-ray findings.
- Goal: Patient will achieve optimal gas exchange as evidenced by improved breathing pattern and reduced breathlessness.
- Interventions: Elevate head of bed, assist with positioning for comfort, administer oxygen as prescribed, monitor respiratory rate, rhythm, and effort, encourage deep breathing and coughing, assess for signs of respiratory distress.
2. Ineffective breathing pattern related to decreased lung expansion secondary to ascites and pleural effusion as evidenced by orthopnea and subjective reports of breathlessness.
- Goal: Patient will demonstrate effective breathing pattern as evidenced by absence of orthopnea and decreased respiratory rate.
- Interventions: Frequent repositioning, abdominal paracentesis and thoracentesis as indicated, administer bronchodilators if wheezing is present, instruct on pursed-lip breathing, monitor fluid balance, implement chest physiotherapy and spirometry as prescribed.
3. Deficient fluid volume (Risk for/Actual) related to active fluid drainage (pigtail catheter) and third-spacing as evidenced by electrolyte imbalances (hyponatremia) and the need for PRBC transfusion.
- Goal: Patient will maintain adequate fluid balance and electrolyte levels.
- Interventions: Monitor intake and output rigorously, assess for signs of dehydration or overhydration, monitor serum electrolytes, administer IV fluids and albumin as prescribed.
4. Acute Pain related to abdominal distension (ascites), mass effect of tumor, and invasive procedures as evidenced by patient’s verbal reports and need for analgesics (Paracetamol, Tramadol).
- Goal: Patient will report pain at a manageable level.
- Interventions: Assess pain level regularly using a validated scale, administer prescribed analgesics (Paracetamol, Tramadol) and evaluate effectiveness, employ non-pharmacological pain relief methods (e.g., relaxation techniques, comfortable positioning), consider patient preference for pain management.
5. Imbalanced nutrition: Less than body requirements related to disease process, malignancy-associated cachexia, and potential gastrointestinal symptoms as evidenced by low albumin and total protein levels.
- Goal: Patient will maintain adequate nutritional intake to prevent further weight loss and improve serum protein levels.
- Interventions: Assess dietary intake, offer small frequent meals, consider nutritional supplements, collaborate with a dietitian, monitor weight and laboratory values (albumin, total protein).
6. Anxiety/Fear related to disease progression, uncertain prognosis, and physical symptoms as evidenced by patient’s decision regarding chemotherapy and need for palliative care.
- Goal: Patient will verbalize reduced anxiety and express coping strategies.
- Interventions: Provide emotional support, active listening, facilitate communication with family and healthcare team, explain procedures and care plan clearly, refer to spiritual care or counselling as appropriate.